There are currently two basic surgery options available for hip replacement – Hip Resurfacing or Total Hip Replacement. Hip Resurfacing is bone conserving and allows a patient to return to an active life with no restrictions. Hip Resurfacing is often used for young male athletes who want to return to the sports they love.
A total hip replacement, THR, has been the gold standard for many years. THR is major surgery that can result in a long post-op recovery and often limits your activities depending on the type of hip device that is used.

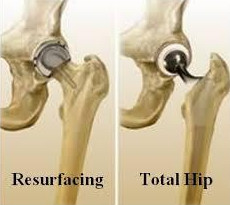
What is a Birmingham Hip Resurfacing or BHR? Why is it different than a total hip replacement? It is bone conserving and removes less bone allowing for a more normal gait after recovery.


Dr. Brooks of the Cleveland Clinic explains what a hip resurfacing is compared to a total hip replacement.
He shows hip resurfacing device compared to a total hip replacement device. He explains how much less bone if removed during a hip resurfacing than a total hip replacement.
Hip Resurfacing devices use a metal cap implanted over the femoral head and a metal cup is placed in the acetabulum to provide a bearing surface. The use of this type of hip device results in a metal on metal bearing and is an excellent choice for hip replacement. The hip resurfacing procedure is bone sparing since it does not require a portion of the femur to be cut off. In a hip resurfacing operation, the surgeon removes only the diseased surface of the head of the femur. A spherical metal cap is fitted over the femoral head. The cap may be placed with cement or be cementless. Different surgeons use different techniques. The hip socket or acetabulum is lined with a thin spherical metal cup and it is not cemented.
The Smith & Nephew Birmingham Hip Resurfacing Device (BHR) was the first device to be FDA Approved in 2006. Several other types of devices like the Biomet, Wright Conserve Plus, Synovo Preserve and new ceramics are available, but not all in the US.
Hip Resurfacing offers the younger, active patient an opportunity to return to a full active life without restrictions and without pain. It is bone conserving. Hip Resurfacing has been the choice of many surgeons world wide for younger people in since 1998
Dec. 3, 2012 in Columbia SC
A total hip replacement means that the top portion of your femur bone will be removed, the remaining bone will be drilled and a device with a long stem will be placed into the bone. The stem is secured with bone cement or secured with a press-fit depending on the technique chosen by the surgeon. A cup will be placed in the acetabulum of the hip to provide a pivoting surface for the ball of the femur device. If you ever need to have a revision, it is much more difficult to removed the stem from the bone after it has been in place for a number of years. The bone must be cut apart to removed the old stem. Revision surgery of a THR is again major surgery and often limits a person in their activities after the revision. There are many types of hip devices and they often dictate what activities are acceptable. The newer, large head ceramic on ceramic hip devices offer a person much more of a very active life style than the older, small metal and plastic hip devices.
There are two types of Hip Resurfacing: a partial (hemi) hip resurfacing or a complete hip resurfacing. The partial hip resurfacing results in a cap being implanted over the femoral head. No cup is placed in the acetabulum to provide a mating pivoting surface. The partial hip resurfacing results in a bone on metal situation. Partial hip resurfacing was the early accepted form of hip resurfacing in the US and was not always successful long term. The metal rubbing on the bone often failed over time. A partial hip resurfacing was used with patients who had osteonecrosis or AVN of the femoral head.
